Glory Casino Bangladesh: Get a Welcome Package with a 125% Bonus
Glory Casino is a respected brand in Bangladesh’s online gambling market. The company offers local players thousands of games and a Welcome Bonus of up to BDT 27,000. Sign up now to play games from top developers and participate in generous tournaments.
GET +125%
ON FIRST DEPOSIT
Copy and use promo code to get bonus

Popular Games

Wild Ape 3258

Aviatrix

Juicy Win: Hold The Spin

3 China Pots

Sun Of Egypt 3

30 Coins

Lucky Streak 3

Juicy Wheel

Luck Of Tiger

Royal Fruits 9

Energy Coins Hold And Win

Pink Jocker Hold And Win
About Glory Casino BD
Glory Casino started operating in the Bangladesh gambling market in 2021. This site is operated by Bettor IO N.V., which has a well-deserved authority in the industry. The casino’s work is subject to international standards, confirmed by a Curaçao license. Only adults can play in it, which aligns with the principles of Responsible Gaming promoted by the company.
 Name
Name
|
Glory Casino |
 License
License
|
Curacao license #365/JAZ |
 Introductory package
Introductory package
|
125% up to 29,000 BDT + 250 casino free spins |
 Support channels
Support channels
|
Live chat, email: [email protected] |
 Mobile availability
Mobile availability
|
Mobile site, Android/iOS application |
 Tools for deposits and withdrawals
Tools for deposits and withdrawals
|
BKash and Nagad |
 Minimum casino deposit sum
Minimum casino deposit sum
|
400 BDT |
 Languages
Languages
|
Bengali, English |
Many positive reviews from Bangladeshi gamblers confirm that Glory Casino is an excellent pick for playing games for BDT. No wonder the company’s customer base is growing.
Is Gambling on Glory Casino Legitimate in Bangladesh?
With a global license from government agencies of Curaçao, the casino is able to lawfully do business in Bangladesh. The company follows all service industry rules and meets guidelines for best practices. Each Glory Casino user can expect instant aid, be it related to a game, bonus, login issues, or anything else. Players have the option to contact the regulator directly to confirm the validity of the permit displayed on the site.

Creating a New Account at Glory – Basic Steps
To access the offerings of Glory Casino, you must create a profile. This process is simple and not time-consuming:
Open the main page of the gambling site or launch the application.
Find the special “Sign Up” button in the top portion of the page.
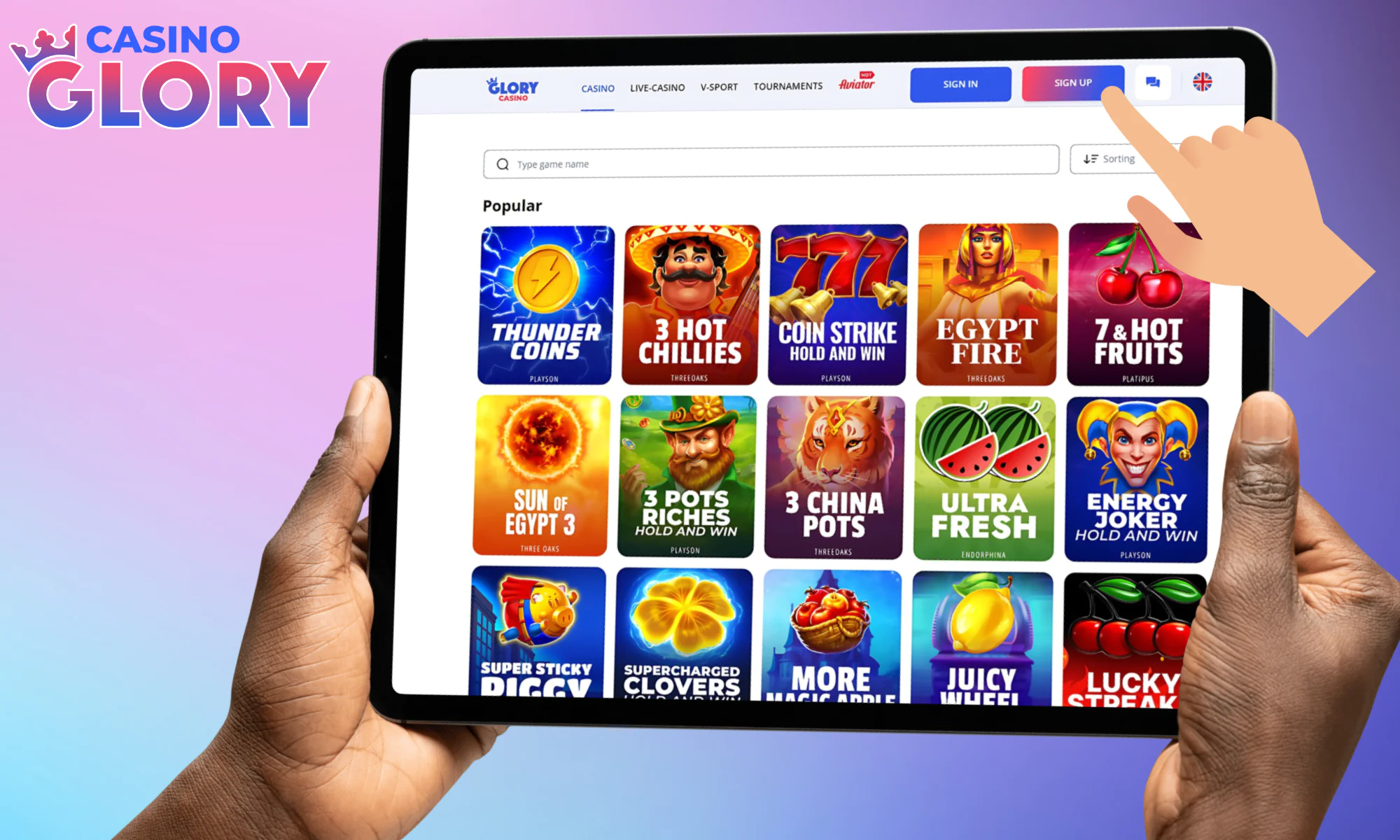
Click on it to see a new window pop up.
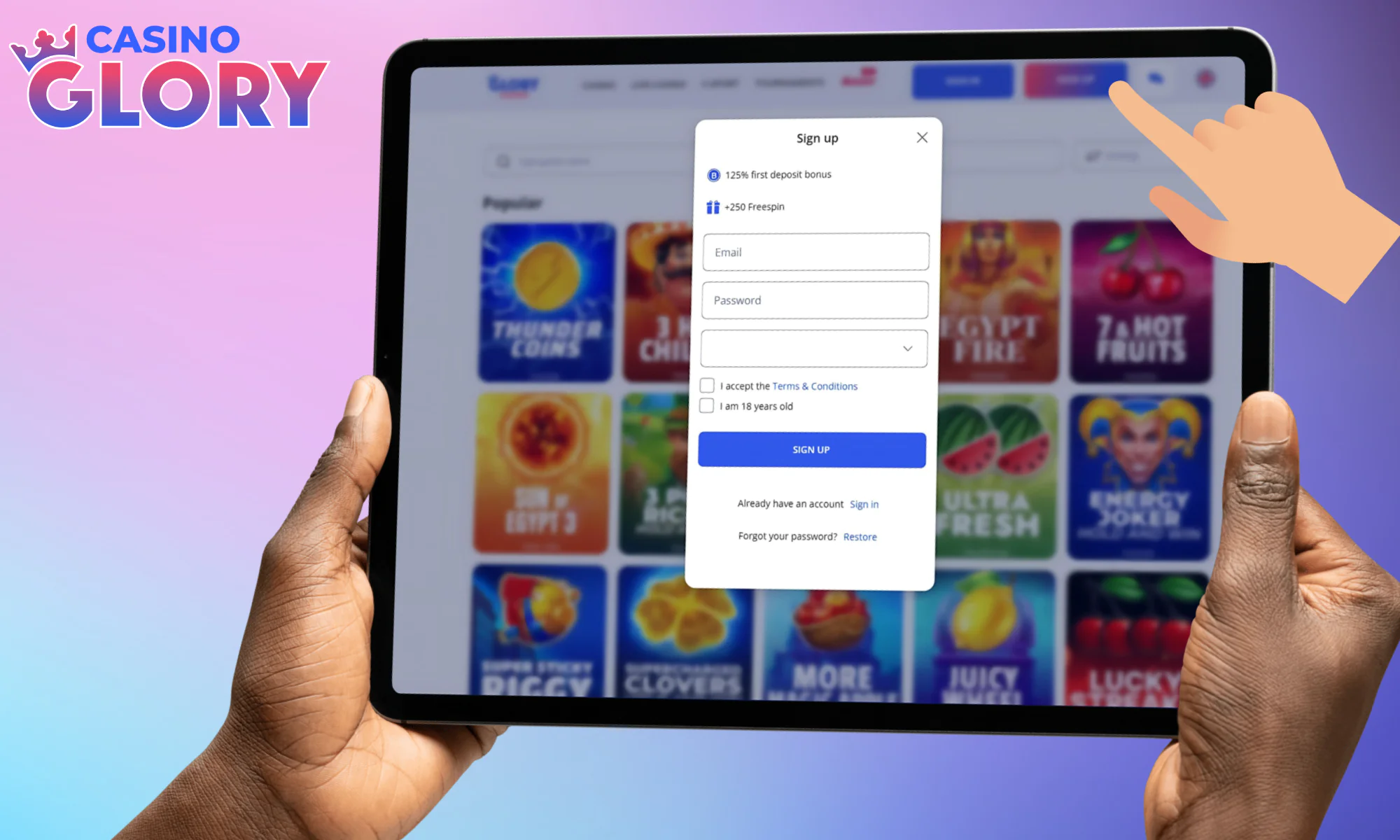
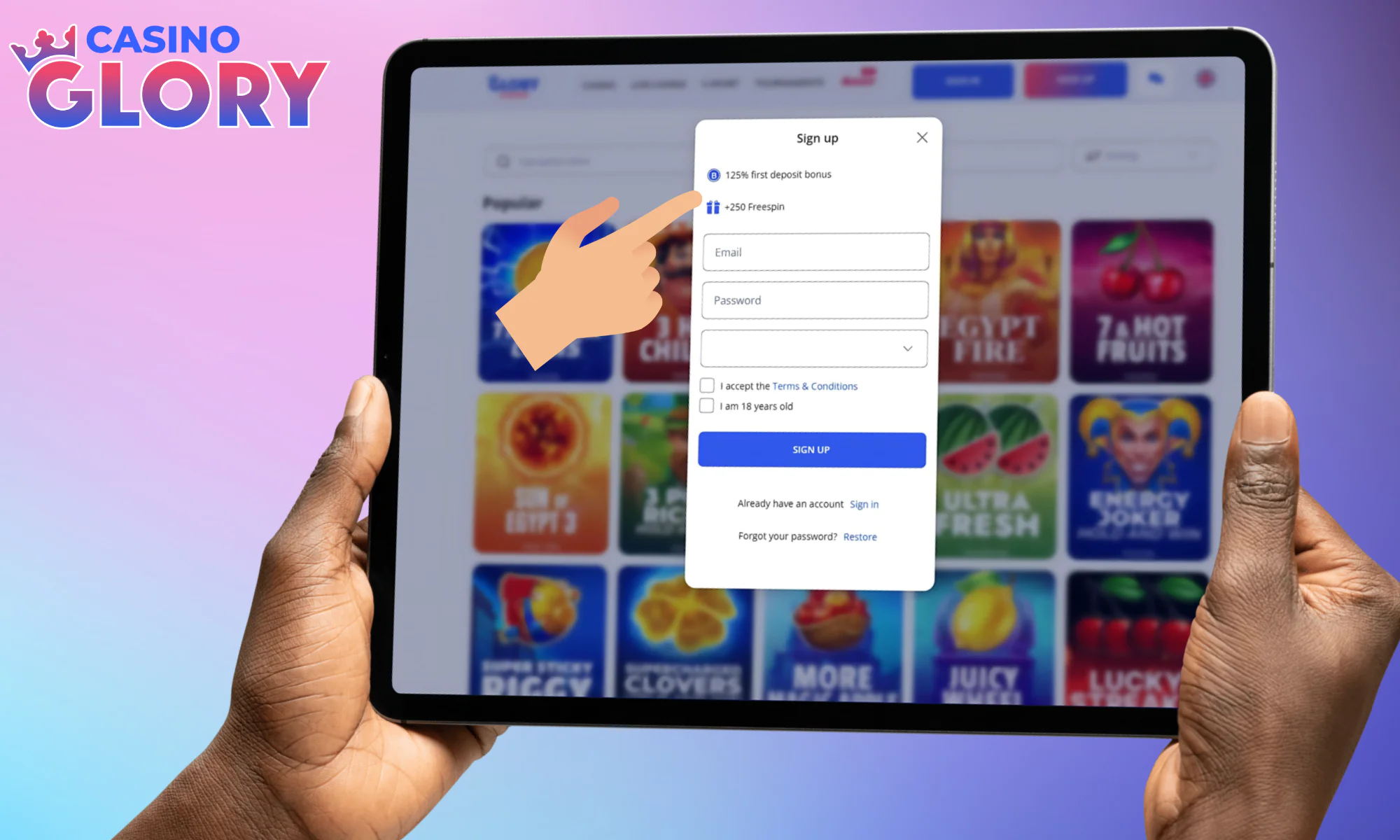
Review the details of your bonus up top.
Input your email, and come up with a good password.
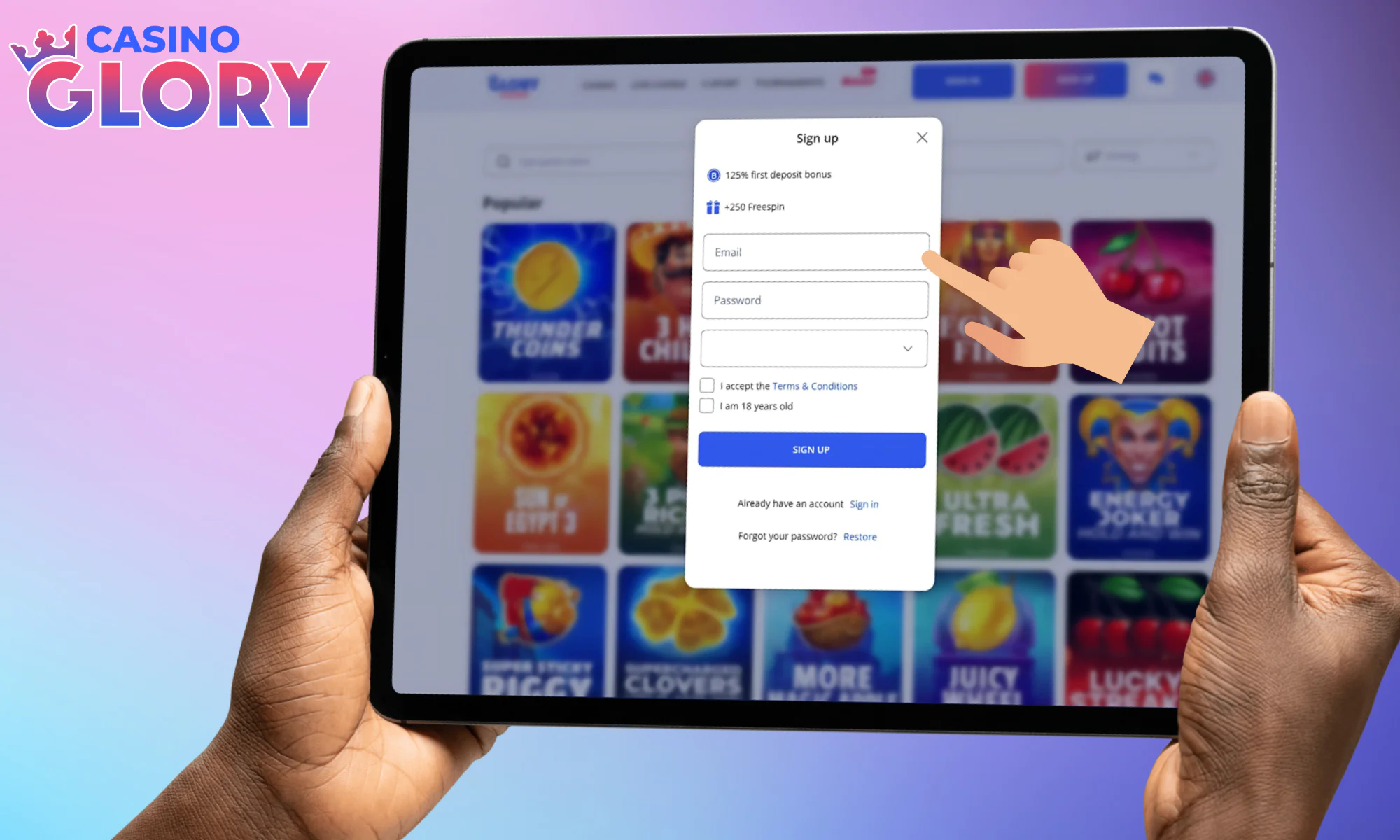
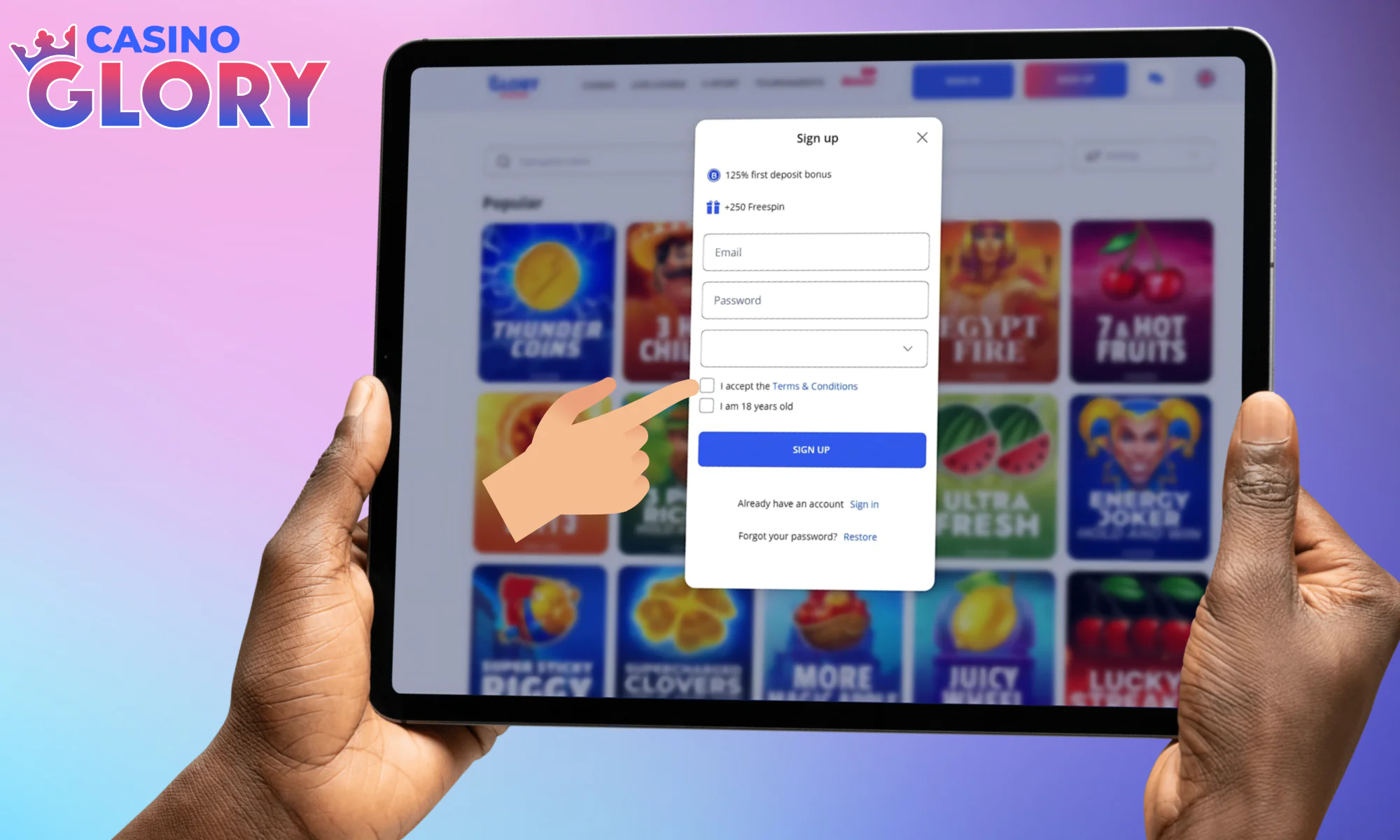
Pick casino BDT as your currency, check all the boxes.
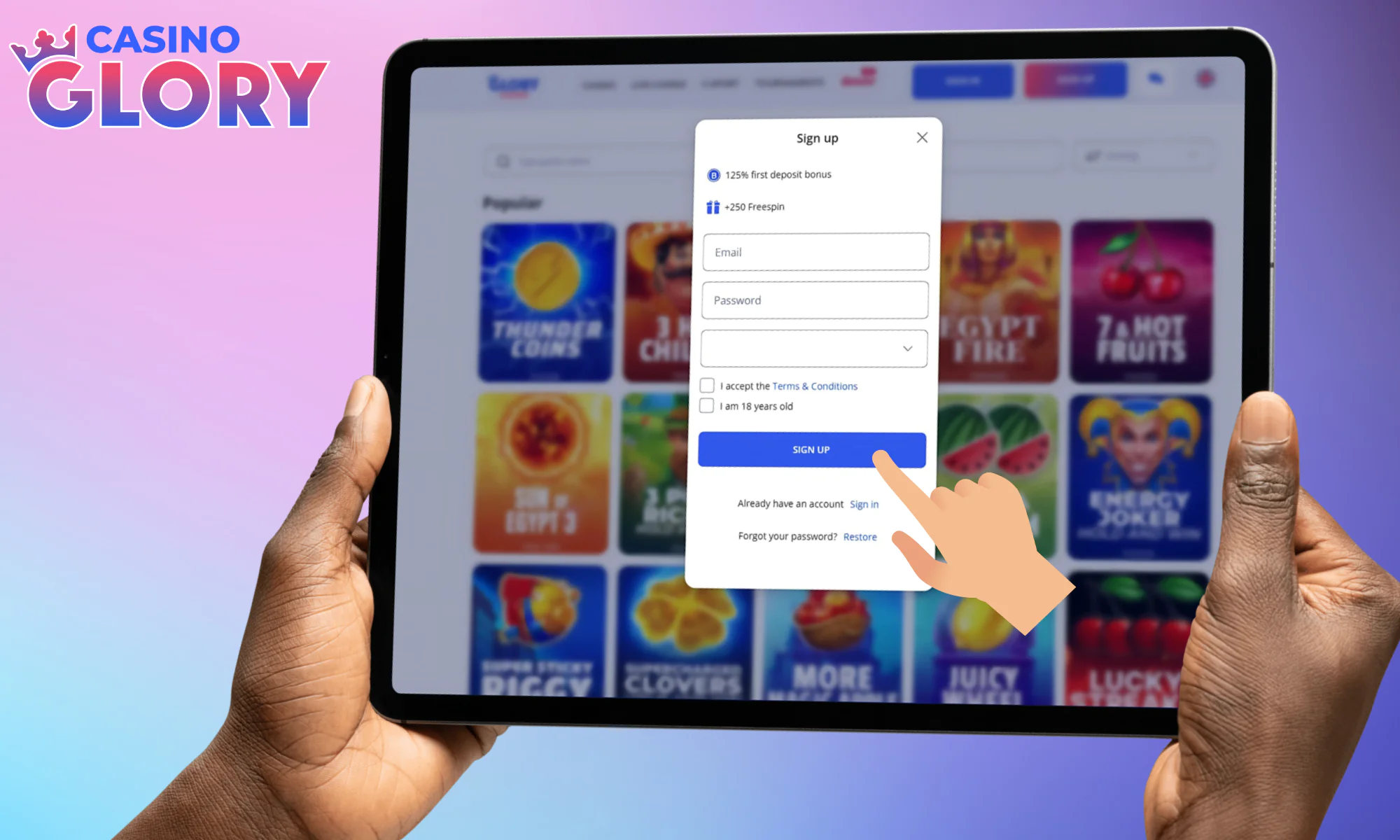
Make use of the Sign Up button to confirm profile creation.
Glory Casino Login
You are free to visit the casino anytime you choose now that your account is live and active. Here is what is required to complete a login:
Open the site or the app.
Use the Sign In button.
Enter your credentials – email and password.
Hit the blue button at the bottom to access the Glory Casino.
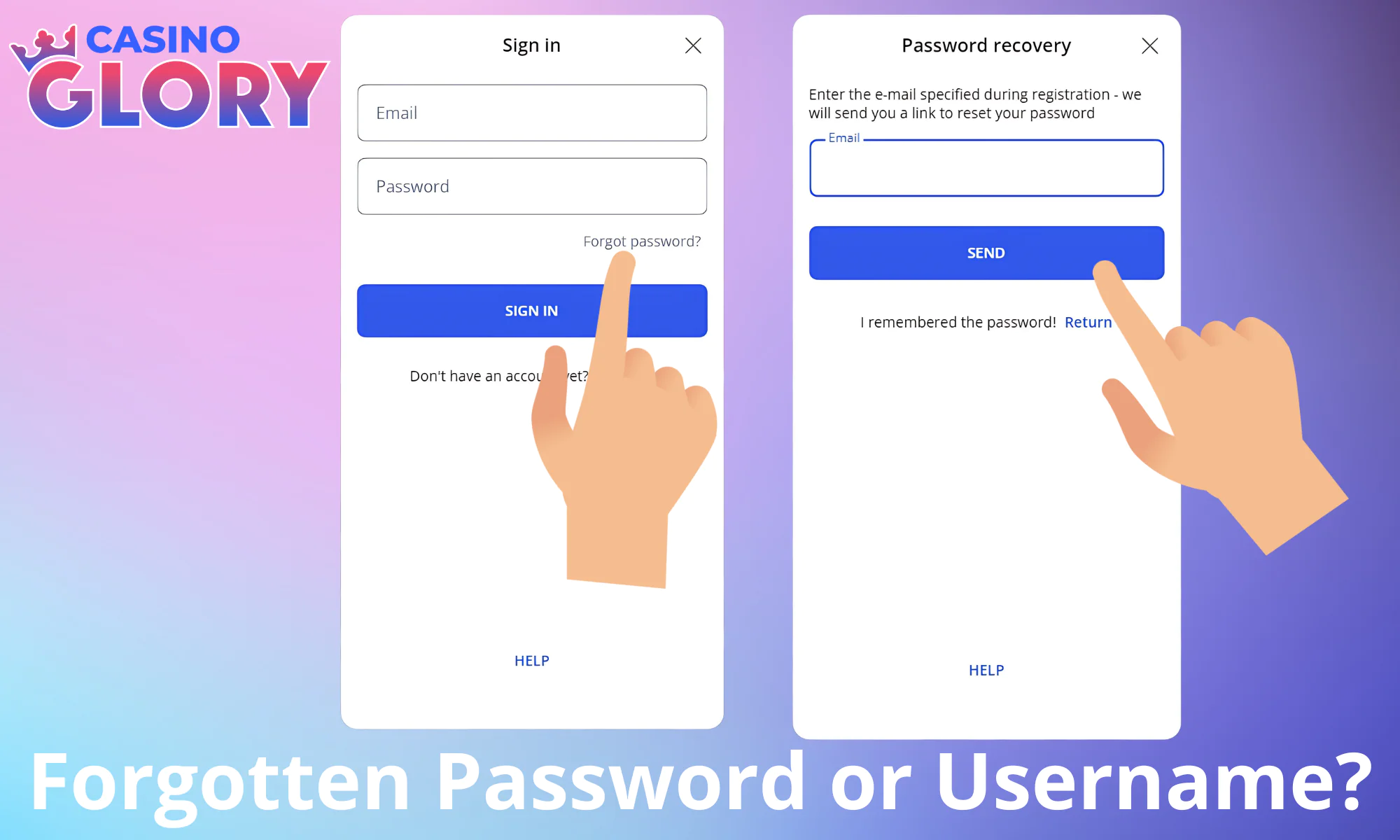
Forgotten Password or Username?
In the event that you are unable to recall your password, the website now includes a “Forgot Password?” button. It is available in the login window. Click it to initiate the username/password recovery process. You will be asked to input the email address used to create your Glory Casino BD account. The email will continue a password reset link so you can access your account as soon as possible.
Why should players trust Glory Casino?
Gamblers playing in Glory Casino can expect quality service and availability of offerings across desktop and handheld devices. Below are the company’s key perks for gamblers from Bangladesh.
Loyalty Program
Glory Casino has a Loyalty Program aimed at active users. Playing games from the site’s compilation on BDT will earn you loyalty points. Once you have accumulated enough points, you will receive a new status and various bonuses.
Instant Payouts
Bangladeshi players can cash out winnings from Glory Casino utilizing bKash, Nagad, and UPay. The company guarantees fast payouts. The standard speed of such transactions is up to 48 hours.
Superior Protection System
Users’ personal data is protected by SSL encryption and stored on separate servers defended by anti-virus and firewalls. The company also utilizes software to track suspicious user activity and hacker attacks.
Games to Suit All Tastes
With over 1,000 games in the Glory Casino library, you’ll never be bored. Bangladeshi gamblers can choose from thousands of amusements with different rules and gameplay nuances, including slots, arcade games, and bingo.
Current Glory Casino Bonuses 2026
Bangladeshi gamblers can take part in various bonuses on the Glory Casino site. The company offers users free spins and bonus cash. Also, regulars have access to tournaments held by the brand in alliance with top game developers.
Quick start with Glory

Discover ancient riches

Grab C600 000!

Chase the Fun And 4000 €!

Welcome Bonus up to 125% +250 FS to the First Deposit
Glory Casino knows that Bangladeshi gamblers are interested in above-average casino rewards. That is why the company provides everyone with a boost of up to 29,000 BDT right from the get-go.
The casino deposit incentive includes a 100% match on your first payment, essentially doubling your initial top-up. There is also an opportunity to increase it by 25%, to 125%, if you top up your casino account within an hour after establishing an account. Additionally, by depositing at least 1,400 BDT, you can unlock 250 free spins.
To activate, simply deposit a minimum of 600 BDT to your balance. There are no additional moves involved, it is triggered automatically. The wager requirements are x50, and you have 48 hours to meet them.

Take Part in Glory Tournaments
To make your game even more potentially lucrative, Glory Casino runs tournaments at all times. The current line-up of tournaments includes:
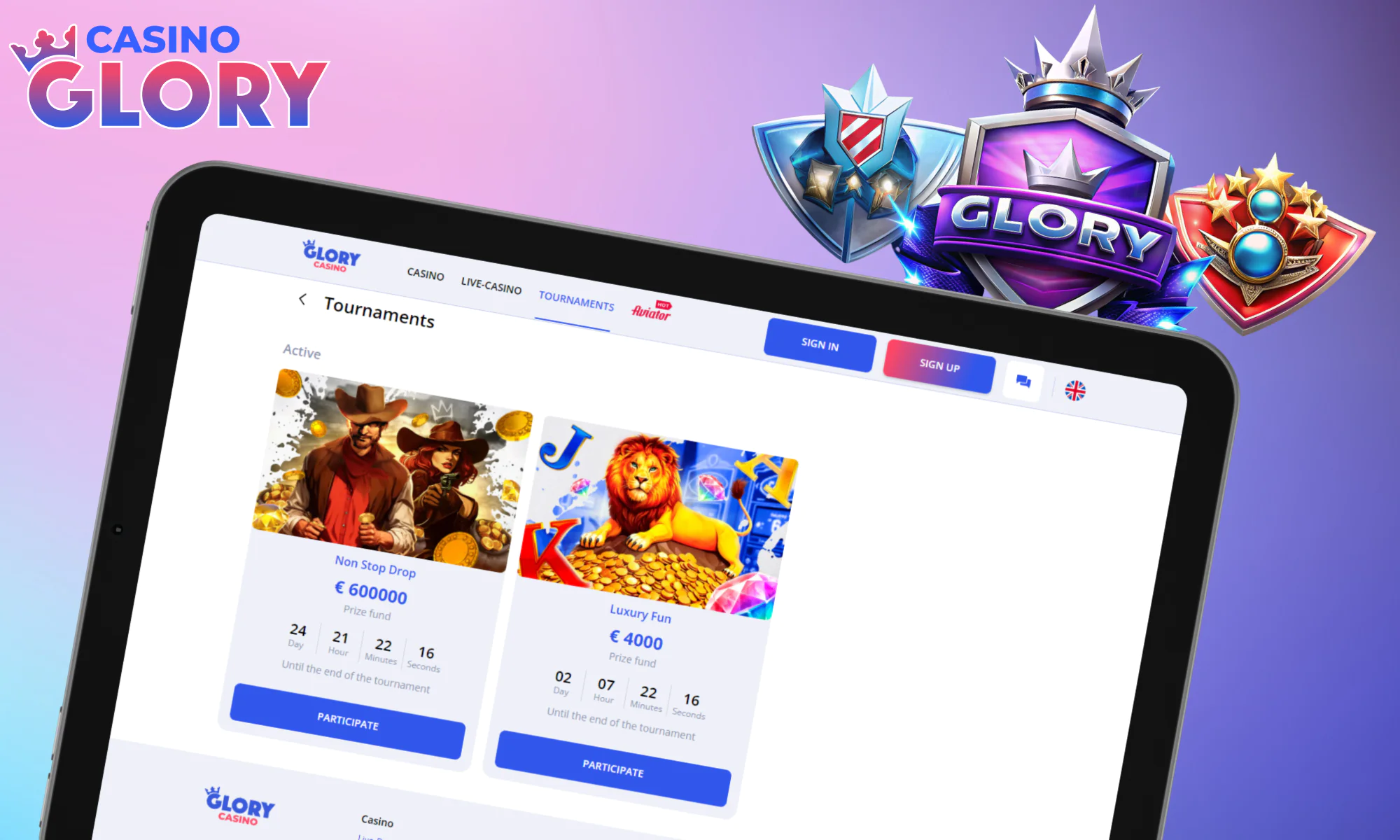
| Casino Tournament Name | Prize Fund | Game List | Description |
| Heart of Gold | 530,000 BDT | Egypt Fire;Sun of Egypt 3;Super Sticky Piggy, and 20+ more. | With a minimum bet of 25 BDT, you can win up to 65,000 BDT. Bonus game features can maximize points earned per game. |
| Non-Stop Drop | 1,850,000,000 BDT | Cash Blast-supporting games | Trigger a random Cash Blast prize with a bet of at least 25 BDT to climb up the ranks. It’s possible to win up to 660,000 BDT with this tournament. |
Remember that the list of online competitions is updated on a frequent basis. To keep up with the changes, you must visit the tournament section on the site or in the app.
Glory Casino Games Library
Glory Casino’s library includes over 1,000 different games. To facilitate players’ ability to choose the most suitable pick, all games are organized into categories. A brief overview is provided in this table:

| Top games | Crazy Time, Aviator, Thunder Coins, Wild West, Book of Mines |
| Categories | Slots, Table Games, Lottery, Poker, Bingo, Arcade, Keno, Instant Games Scratch Games, Roulette, Blackjack |
| Providers | Playson, Spribe, Pragmatic Play, Betsoft, Platipus, and 100+ companies |
Slots Collection
‘Slots’ is the most game-rich tab in the Glory Casino compilation. It features several thousand amusements, and the number of them is constantly increasing. Here are some examples of the most popular slots among Bangladeshi gamblers:
- Big Bass Mission Fishin’ Mobile;
- Fulong 88;
- Bouncy Bombs;
- Land of the Free;
- Golden Era;
- Thunder Coins;
- Egypt Fire;
- Mega Flip.
Gamblers can pick from different types of such amusements: classic slots, video slots, progressive and fixed jackpot games. Slots with additional gameplay mechanics, such as Megaways and Hold & Win, are also available.

Exclusive Games
One more thing that sets Glory Casino apart is the games developed by an in-house team. These one-of-a-kind slot machines and other games are designed with local preferences in mind. These games often include new features such as:
- Expanding wilds;
- Cascading reels;
- Multilevel in-game bonuses.
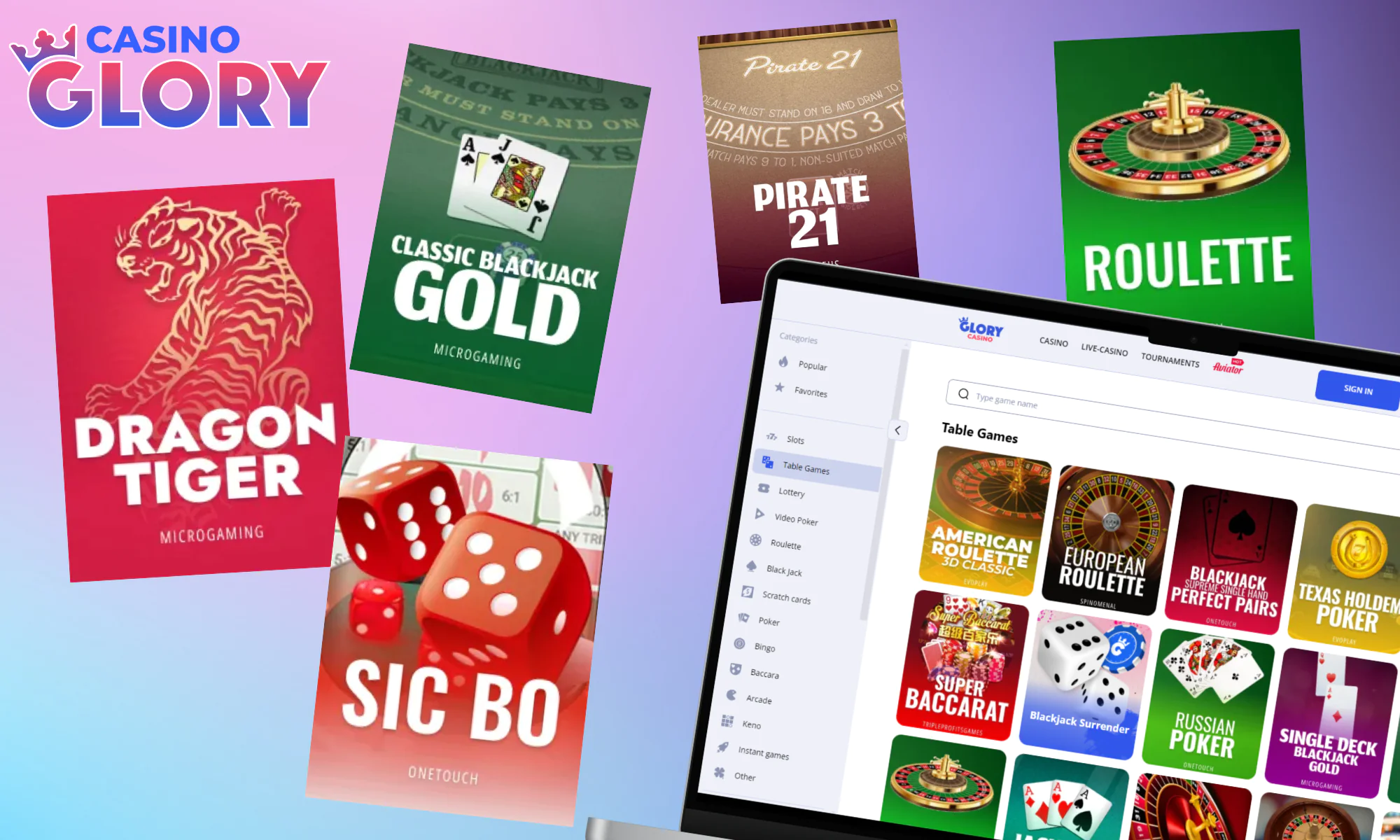
Table Games
For fans of traditional gaming, Glory Casino offers a robust selection of table games. Whether you prefer strategy or luck, or a mix of both, there’s a choice for you. Enjoy timeless classics such as blackjack, roulette, and baccarat. Play such in-demand titles as:
- Dragon Tiger;
- Sic Bo;
- Classic Blackjack Gold;
- Jacks or Better;
- Roulette;
- Pirate 21.
Arcade
The Gambling company also boasts a variety of arcade games. 10+ picks bring back the good old days of playing in a real arcade, perfect for those who want a change of pace from the usual casino fare. Space Invaders and Pac-Man-style titles are available. Among the most popular picks, there are:
- Darts Champion;
- Blast Man;
- Thunder Land;
- Deluxe Dice;
- Tap Heroes;
- Plinko S.

Bingo
Bingo is a great choice, that can provide hours of fun whether played alone or with others. There are plenty of variations that give a little something more to keep the action interesting: 75-ball, 80-ball, and 90-ball. The top picks are:
- Zodiac Bingo;
- Beto Bingo;
- Halloween Bingo;
- Bingo Iglu;
- Whale Bingo;
- Bingo Royale.
Keno
As with other lottery-style games, Keno places a heavy emphasis on chance. Pick some numbers, sit tight, wait for the draw, and collect your winnings if they match. The most in-demand Glory Casino keno picks are:
- Keno Universe;
- The Candy Keno;
- Across the Universe Keno;
- Keno Universe;
- Keno Jackpot;
- The Candy Keno;
- Burning Keno.

Live Games
Glory Casino’s live game section delivers high-definition, real-time gaming with expert dealers, creating the illusion of being at a physical casino. It is perfect for anyone looking for an authentic gambling experience.
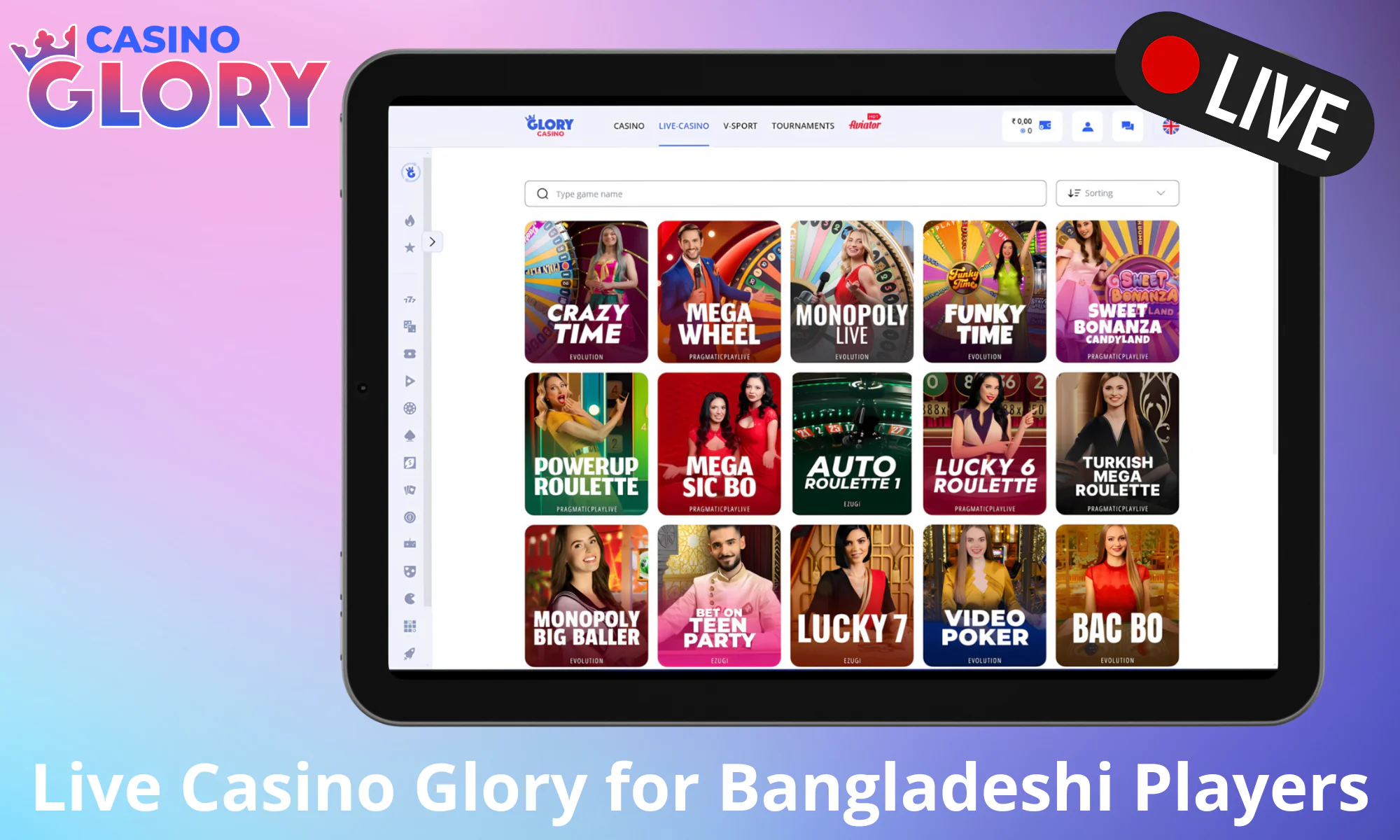
Roulette
You may readily play European, American, and French Roulette, among others, at Glory Casino BD. Rules and core gameplay differ a lot in each variant, but there’s common ground too. Don’t just test your luck blindly, manage your bets using a strategy like the Martingale or the D’Alembert to improve your odds of winning across in-demand picks such as Casino Marina Roulette 2, Auto Mega Roulette, or CA1 Roulette.
Blackjack
Yet another popular live option is blackjack, a game that combines skill and chance. The rules and techniques for the countless variations are different, but Glory Casino offers them all. Get as near to 21 as you can without going over. It is always changing because of the importance of strategy and chance.
Poker
At Glory Casino, you may find 38 different games, including such hits as Russian Poker, Bonus Deuces Wild, and Three Card Poker. Every player is encouraged to take part in playing well-known variants such as Omaha and Texas Hold ’em. Rules and techniques vary throughout to accommodate various playing styles. In addition to the live selection, there is also a stand-alone category for video poker.
Baccarat
There is a stand-alone category for baccarat as well. Make a bet on who will have a hand closer to nine: the player or the banker. Knowing the odds can help in making informed bets. You may change up your game experience by trying out variations like Chemin de Fer or Punto Banco.
Quick Games
Playing instant games at Glory Casino online is a great way to both have fun and potentially enjoy rapid wins. If you’re looking for a quick play that won’t take long to play and has a high RTP, try out any of the options mentioned below.
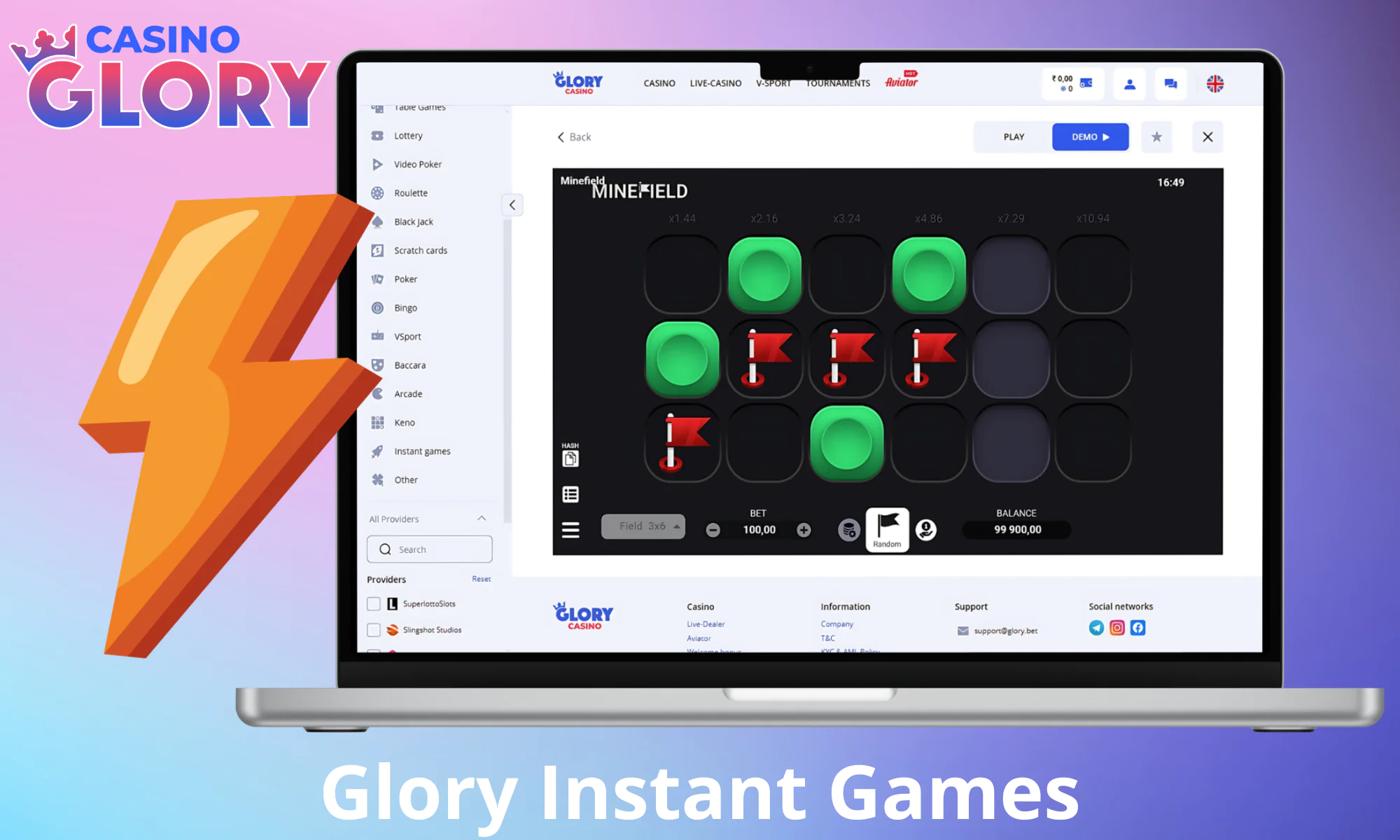
Aviator
Bet on an airplane’s flight path in Glory Casino Aviator, a hit Spribe release. Your payouts increase as the plane gains altitude but so are the chances of losing your entire stake whenever this jet speeds off offscreen. The goal is to collect your money by cashing out in time. The maximum multiplier of x1,000,000, ease, and provable fairness of Aviator make it very well-liked in Bangladesh
Plinko
Plinko involves dropping a chip down a pegged board to determine your prize. It’s a simple game of chance with rules that everybody can easily grasp. Unlike Aviator, it’s not a stand-alone release, but a category – there are 15 Plinko games in total, including Golden Plinko, Plinko X, and Bonus Mania Plinko. Quick wins can be yours for the taking across all the high-RTP options presented
Mines
Glory Casino players take risks by avoiding mines as they uncover grid squares to show reward multipliers. This subgenre is based on Minesweeper. You lose if you uncover a mine, and can typically cash out any time to introduce some strategy into your gaming experience. Players have the option to reveal a small number of squares or to aggressively seek higher multipliers.
Limbo
Limbo is a simple yet thrilling game where players set a multiplier and win if a generated number is higher. There are only 3 limbo games currently available – Limbo XY, Limbo (Turbo Games), and Limbo from GloryCasino. This game’s simplicity and potential for big rewards appeal to many players.
Software Providers
Top best developers provide their games to the site’s collection. Glory Casino partners with 100+ companies, including Spinomenal, BetSoft, and Red Tiger.
-

Pragmatic Play
-

Endorphina
-

Hacksaw Gaming
-

Mascot Gaming
-

Spinomenal
-

Playson
-

Nolimit City
-

Platipus
-

Red Tiger
-

3 Oaks Gaming
-

PG Soft
-

Blueprint Gaming
-

Evoplay
-

Netgame
-

BGaming
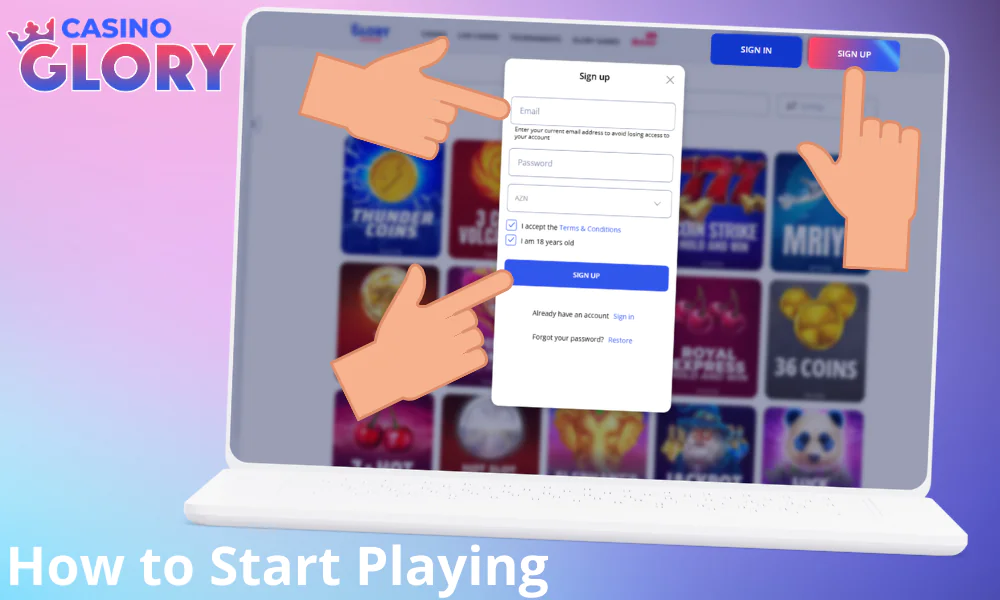
How to Start Playing Glory Casino: A Detailed Information
Every user from Bangladesh can play in Glory Casino. If you are of legal age, then follow this sequence of actions:
- Create a profile. You must have a profile to play for BDT, so register.
- Fund your balance. Click on the wallet icon in the top menu and pick a payment tool (bKash, Nagad, or Upay) to make a deposit.
- Launch the game. Now open the ‘Casino’ or ‘Live Casino’ tab. Pick a game and launch it in the paid version.
- Play. Place a bet and play.
Glory Casino instantly credits winnings to players’ balances, and they can withdraw them after verification
Common Gambling Mistakes to Avoid at Glory Casino
It is essential to learn the most prevalent casino pitfalls and how to avoid them before placing real money bets in any of the Glory Casino games. The most prevalent casino mistakes are:
- Overlooking the Rules. You can win more often in any casino game by learning and sticking to the rules. To improve your casino play, you may even check out outside game tutorials and guidelines.
- Risking More Than You Can Afford. Avoid financial hardship by forming and adhering to a budget. Casino game playing should be more about having fun, not trying to win big.
- Chasing Losses. Do not chase your casino losses by betting more. Deeper losses and more risks are the most common results of impulsive casino betting.
- Playing a Game When You’re Weary or Under the Influence. Decisions made under the influence of alcohol or while too tired are never rational.
- Neglecting to Take Regular Pauses. Taking pauses when playing any casino game allows you to stay focused and avoid fatigue. This, in turn, improves your game experience and might even lead to more frequent wins.

Glory V-Sport – New Section on the Official Website
For casino fans who also have an interest in sports betting, Casino Glory has introduced a fresh game category: V-Sports. Here, you can bet on virtual simulations of sports matches with real teams across different sports. The casino game category contains 33 options at the moment, including:
- World Cup on Demand;
- Horses & Flat on Demand;
- Colombia League;
- Euro 2020.
Each game is accessible 24/7 for your casino bets, and there are no off-seasons.

Mobile Application by Glory Casino
All Glory Casino services are available on desktop and mobile devices. If you prefer to play games on your smartphone or tablet, you have that chance. The company has an mobile app for Android/iOS gadgets and a mobile version of the site. Both options have their advantages.
Mobile Application
The Glory Casino software is accessible in two versions — for Android and iOS. You must download and install it from the company’s site to utilize it. Here are the advantages of the program:
- Download and installation are free;
- The application takes only a few megabytes in the memory of the gadget;
- Powerful data encryption;
- The application is optimized to be energy-saving;
- Ability to customize notifications of new games and bonuses.
Be sure to update the Glory Casino app to the new version as soon as it is available. You may encounter lags, bugs, and vulnerabilities in the software’s security.

Browser Version
As an alternative to the app, gamblers from Bangladesh can use the mobile variation of the Glory Casino site. Here are the perks of this option:
- You don’t need to download and install anything;
- The site does not require any free space in the memory of the gadget;
- You can use any browser to access it;
- No technical requirements;
- The site is adaptive and adjusts to your smartphone screen.
Also, gamers can use the same login and password as on the desktop. You do not need to register a second time.

Glory Payment Methods in Bangladesh
The casino has opened its doors to players from BD and made sure that their transactions are safe and secure. So, you may avoid extra fees and conversions by making deposits in Bangladeshi taka. Even though Glory Casino does not have the biggest line-up of payment tools, all of them are considered to be very safe and were handpicked due to speed and accessibility:
| Casino Payment Tool | Deposit Limits | Withdrawal Limits |
| BKash | 400 – 25,000 BDT | 400 – 25,000 BDT |
| Nagad | 400 – 25,000 BDT | 400 – 25,000 BDT |
Ways to Contact the Support Team
Get in touch with Glory Casino user service representatives whenever it is most convenient for you. They are available 24/7 for all Bangladeshi users. Whenever you have a problem with a specific game or have a query related to device errors, types of cashback offers, a casino agent will be there to help you out. Feel free to get in touch via any of the following channels:
| Live casino chat | Use the dialogue icon at the top of the page, the average response time is under 2 minutes. |
| Social Media | Links to active Telegram, Instagram, and Facebook accounts of Glory Casino are provided in the footer |
| You can send a message to [email protected] to receive a timely response |

FAQ
Do I Need To Register A Second Account To Play On Mobile?
No. The same account is used to access both the Glory Casino website and the mobile app/site version. All your data carries over as well.
Are All Glory Casino Games Available In Demo Mode?
No. No game from the live casino category is available in trial mode. Even though you can observe these games as a spectator.
Is There A Way To Ensure Continued Winning In Glory Casino?
No. Every game on the site is verifiably random and there’s no way to always win in games of chance.
Will There Be New Payment Instruments Introduced?
Most likely – yes. Glory Casino can introduce new tools for payments as well as additional promotional offers in the future.
What Are The Most Popular Casino Games From The Lottery Category?
The most in-demand game here is Scratchy Big. Other trending options include Lucky Number x16 and Cash Vault 1.
Is It Possible To Self-Exclude At Glory Casino?
Yes. Be sure to read the company’s Responsible Gambling policy (accessible from the footer) before sending in an email to the specified address.
Is The Download And Installation Of The App Necessary To Play On Mobile?
No. You can use the regular Glory Casino mobile site to play. You are not missing out on any features on the casino side, but it may not be possible to enjoy additional perks your operating system provides for the app, including integrational features.
Last updated: